Which Statement About Creb327 Phosphorylation Is Supported By The Data Presented In Table 1?
- Inquiry
- Open Access
- Published:
Neurodegenerative phosphoprotein signaling landscape in models of SCA3
Molecular Brain volume xiv, Article number:57 (2021) Cite this article
Abstruse
Spinocerebellar clutter type 3 (SCA3) is a rare neurodegenerative disorder resulting from an aberrant expansion of a polyglutamine stretch in the ataxin-three poly peptide and subsequent neuronal death. The underlying intracellular signaling pathways are currently unknown. We applied the Opposite-phase Protein MicroArray (RPMA) technology to assess the levels of 50 signaling proteins (in phosphorylated and full forms) using three in vitro and in vivo models expressing expanded ataxin-3: (i) homo embryonic kidney (HEK293T) cells stably transfected with human ataxin-3 constructs, (ii) mouse embryonic fibroblasts (MEF) from SCA3 transgenic mice, and (iii) whole brains from SCA3 transgenic mice. All iii models demonstrated a high caste of similarity sharing a subset of phosphorylated proteins involved in the PI3K/AKT/GSK3/mTOR pathway. Expanded ataxin-3 strongly interfered (by stimulation or suppression) with normal ataxin-3 signaling consistent with the pathogenic role of the polyglutamine expansion. In comparison with normal ataxin-3, expanded ataxin-iii caused a pro-survival stimulation of the ERK pathway forth with reduced pro-apoptotic and transcriptional responses.
Introduction
Spinocerebellar ataxia type 3 (SCA3), likewise known as Machado-Joseph affliction (MJD), is an autosomal dominantly inherited ataxia which is characterized past deficits in gait, move, and coordination linked to a CAG repeat expansion in the ATXN3 gene and a concordant polyglutamine expansion in the ataxin-3 protein. Therefore, SCA3 falls into the group of polyglutamine disorders which includes Huntington'due south illness and within the broader category of neurodegenerative disorders to include Parkinson's disease and Alzheimer'south illness [55].
Although the gene responsible for SCA3 was discovered more 25 years agone [25], the cellular signaling pathways of neurodegeneration remain elusive. Protein activation mapping and identification of critical nodes in the signaling network represent fundamental approaches required to elucidate the underlying nature of the disease and for the evolution of effective therapeutic interventions. Traditional depression-throughput methods of protein analysis such equally Western blotting and immunohistochemistry take served an of import role in understanding SCA3, but their well-known limitations include difficulty in analyzing complex signaling networks at the level of protein expression and post-translational modification. On the other mitt, Dna microarrays and RNA sequencing provide readouts restricted to the mRNA level with little follow-up on how these changes manifest at the protein level. While RNA encodes information nearly cellular condition, proteins are the ultimate drivers of the cellular machinery serving in fundamental machinery of cell signaling through mail-translational modifications. Reversible protein phosphorylation, especially on serine, threonine or tyrosine residues, is one of the most important and well-studied mail-translational regulating protein part and point transmission [5]. However, phosphorylated proteins are notoriously hard to analyze on a mass scale past traditional methods such every bit Western absorb particularly when small amounts of samples demand to be tested with high sensitivity.
To that terminate nosotros utilized the Reverse-phase Protein MicroArray (RPMA) engineering, a sensitive, quantitative, and high-throughput immunoassay to clarify protein and post-translational modification at the level of total poly peptide abundance or specific phosphorylation. In the RPMA analysis a few microliters of cell lysates are printed onto nitrocellulose slides which are then probed with the poly peptide-specific antibodies. The amount of leap antibody is quantitated using a highly sensitive colorimetric or fluorometric procedure [60].
Here, we present an RPMA-based analysis of three SCA3 models including homo embryonic kidney (HEK293T) cells stably-transfected with plasmids encoding different ataxin-3 variants with normal (xv glutamines, HEK15Q) and expanded polyglutamine repeat (148 glutamines, HEK148Q), mouse embryonic fibroblasts (MEF) from ataxin-3 148Q (MEF148Q, [12]) and ataxin-three knockout mice (MEFKO, [44]), and whole-brain samples from a SCA3 mouse model (CamKII/SCA377Q, [11, 43, 48]). Our data provide assessment of the phosphoprotein signaling mural contributing to the development of the illness phenotype in the context of different SCA3 models and suggest that the RPMA engineering science can exist broadly practical to characterize neurodegenerative disorders thus assisting in biomarker identification and developing novel targets for therapy.
Materials and methods
Cell and Mouse models of SCA3
HEK293T cells were stably transfected with a plasmid expressing green fluorescent protein (GFP)-tagged ataxin-3 with xv glutamines (HEK15Q), ataxin-three with 148 glutamines (HEK148Q), or empty GFP plasmid (HEKempty) as control. Transfected cells were sorted by FACS and highly expressing cells were collected for analysis [48]. Mouse embryonic fibroblasts (MEF) were isolated as described in Hübener et al. [24] from the ataxin-3 148Q (MEF148Q), ataxin-three knockout (MEFKO), and wild-type mice (MEFwt). The ataxin-iii knockout mouse line is described in Schmitt et al. [45] and the ataxin-3 148Q mouse line was generated as described in Boy et al. [12]. C57BL/6 wild-type mice were used to isolate command fibroblasts. The CamKII/SCA377Q mouse model is described in Schmidt et al. [44]. Mice were sacrificed at 12 months of age.
RPMA assay
For Reverse-phase Protein MicroArray Analysis (RPMA, [60]) we used an established and pre-validated prepare of 50 different antibodies [38, 39] directed confronting the total and phosphorylated forms of signaling proteins which encompass a wide array of signaling pathways relevant to the cell fate including apoptosis, survival, and autophagy (Additional file 1: Tables S1 and S2). All antibodies were validated for specificity prior to testing. RPMA assay was performed according to Einspahr et al. [17]. Samples were printed onto the array slides at concentrations 100%, 50%, 25%, and 12.5% in duplicates and prepared for staining past treating with Reblot (Chemicon, CA). Slides were treated with blocking solution I-block (Applied Biosystems, MA) at 2 g/l and 0.5% Tween-20 in PBS. The full poly peptide amounts loaded on the chip were estimated using SYPRO Ruby Protein Blot Stain (Invitrogen, CA) co-ordinate to the manufacturer's instructions and imaged on the NovaRay scanner (Alpha Innotech, CA). Blocked arrays were stained with antibodies on an automated slide stainer (Dako, CA) using the Catalyzed Signal Amplification Arrangement kit according to the manufacturer's recommendation (Dako, CA). A signal was generated using streptavidin-conjugated IRDye 680 (LI-COR Biosciences, NE). Stained slides were scanned on the NovaRay scanner. The TIF images of the antibody and SYPRO-stained slides were analyzed using MicroVigene v2.ix.9.9 software (VigeneTech, MA). Briefly, MicroVigene performed spot finding, local background subtraction (using local and slide average intensity), replicate averaging and total protein normalization, producing a single value for each sample at each dilution. All point values produced for data analysis were at least ii standard deviations in a higher place background. All four dilutions of each sample were analyzed for linear regression and only measurements which met linearity were kept for assay. For each sample and antibody, SCA3 samples were normalized to the corresponding poly peptide concentration in the wild-type sample at the same dilution and averaged beyond all bachelor dilutions. These were and then analyzed past the t-exam to determine the p-value for each averaged protein level. All differences compared to wild-type found pregnant with 95% confidence are shown in Boosted file 1: Table S1.
Western blot analysis
Western absorb analyses were substantially performed every bit described previously [48, 56] with 30 µg of protein loaded from each sample on Tris–glycine or Bis–Tris SDS polyacrylamide gels and separated electrophoretically. Afterwards, proteins were transferred to 0.ii µm nitrocellulose membranes (GE Healthcare, Dornstadt, Germany) using the respective transfer buffer. Afterwards blocking, the membranes were incubated with the same master antibodies as used in the RPMA analysis (for details and dilutions see Additional file one: Table S2) diluted in TBST at 4 °C overnight. Post-obit the incubation with a fluorescence- or HRP-labeled secondary antibiotic, membranes were detected using an Odyssey Fc Imager (LI-COR Biosciences, Bad Homburg, Germany) and quantified using the Image Studio Software (LI-COR) or ImageJ. Statistical analyses were performed using GraphPad Prism viii.40 for Windows (GraphPad Software, San Diego, CA). Data is shown as arithmetic ways ± SEM. Outliers were identified using Grubbs' exam with blastoff = 0.05. Statistical significance of data sets was determined using Student's t-exam and p-values less than or equal to 0.05 were considered as statistically significant.
Results
There is mounting testify that inflammatory signaling cascades are involved in the evolution and pathogenesis of SCA3 [4, xv]. We thus generated a snapshot of the SCA3 degenerative landscape via a large screen of phosphorylated signaling proteins which act as chief regulators of signal transduction beyond multiple signaling networks. The listing of tested proteins and their levels relative to corresponding controls are presented in Additional file 1: Tabular array S1.
Our results show that the overexpression of both normal and expanded ataxin-three has a profound issue on several signaling pathways. Of significant notation is the phosphatidyl inositol three-phosphate kinase (PI3K) cell survival pathway. Phosphorylation of its fundamental members (Table 1) is robustly contradistinct.
Major signaling responses altered by expanded ataxin-iii in SCA3 models
Although nosotros practical a wide range of cellular and beast models, the sets of altered signaling proteins in response to expanded ataxin-3 were establish to exist rather similar (Fig. 1a). Pairwise comparison of the HEK148Q and MEF148Q cell responses in spite of the dissimilar species origin (man vs. mouse) revealed just four proteins as not-shared low responders (with levels changed by less than ± 0.1). As expected, encephalon of the SCA3 transgenic mouse model was more afar from the cultured cells: 16 responses were found unique for the MEF148Q cells or the analyzed brains. All three models shared signaling nodes of major cell pathways important for cell fate (Tabular array 1). A fragment of the signaling network illustrating these results is shown in Fig. 1b. Comparison of the HEK15Q and HEK148Q cells identified changes due to the polyglutamine expansion within ataxin-three (Additional file 1: Tabular array S1). Among the prominent differences (above the ± 20% threshold) between these cells the polyglutamine expansion caused reduction of the levels of apoptotic and transcriptional regulators (Table one). The increased responses included the pro-survival ERK-p70S6K pathway (Table 1).
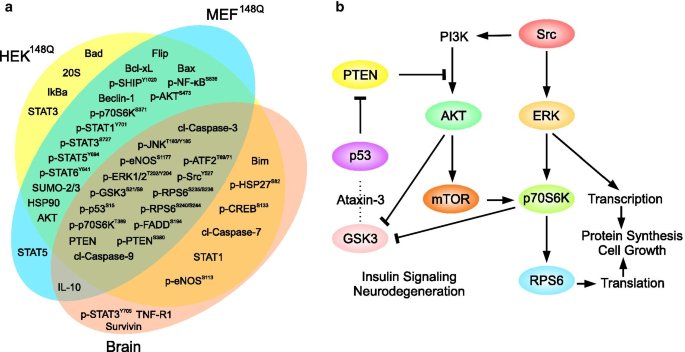
Altered signaling proteins in models of SCA3. a Signaling proteins responding to expanded ataxin-3 expression in the indicated SCA3 models (HEK148Q, MEF148Q and brain of SCA3 mouse model) in comparison to the respective controls (HEKwt, MEFwt, and command brain). In all three different SCA3 disease models, we observed a significant overlap of dysregulated signaling proteins. For clarity, just the proteins displaying alterations of more that ± 20% relative to the corresponding control levels are shown. b A fragment of the signaling network illustrating connections between shared proteins in a
Effect of wild-type ataxin-three and the polyglutamine expansion
To evaluate the contribution of normal (non-expanded) ataxin-3 in the jail cell signaling we analyzed cells lacking ataxin-three (MEFKO) in comparing with wild-type cells (MEFwt). The proteins were grouped together based on their stimulation or suppression relative to the wild-type cells (Fig. two). It is typically causeless that the proteins in the knock-out (KO) cells would brandish contrary effects (suppression vs. stimulation) compared to wild-type cells. We therefore compared the reaction towards the loss of ataxin-3 (MEFKO vs. MEFwt) with the reaction induced by the polyglutamine expansion inside ataxin-3 (MEF148Q vs. MEFwt). Effigy 2 shows that a number of responses induced by the presence of expanded ataxin-three were suppressed by the presence of wild-type ataxin-3 (stimulated in MEFKO cell). On the other paw, a large group of responses including the major cell fate proteins were stimulated past the presence of wild-type ataxin-3 (suppressed in MEFKO cells) but suppressed in cells expressing expanded ataxin-3. No responses stimulated by wild-type ataxin-3 were found to be further amplified by the presence of expanded ataxin-3. Equally expected, the SCA3 whole brain samples demonstrated a certain caste of specificity compared to the MEF148Q cells (Fig. 2). In spite of these specifics the results from both models demonstrated that the expanded ataxin-3 strongly interfered with the normal ataxin-iii signaling.

Side-by side comparison of signaling in reaction to the presence of wild-type and expanded ataxin-3 in MEF cells (a) and SCA3 mouse brain (b). Shown are the relative poly peptide levels of signaling proteins altered upon the presence of wild-type ataxin-3 (comparison of MEFKO with MEFwt) or expanded ataxin-3 (MEF148Q vs. MEFwt) in MEF cells (a) also as in mouse brain (SCA3 vs. wt mouse brain, b). Proteins are grouped according their stimulation (↑) / suppression (↓) by wild-type (ataxin-3wt) and expanded ataxin-3 (ataxin-3exp). For clarity, only the protein pairs displaying alterations of more that ± xx% in one or both proteins relative to the respective control levels are shown
To corroborate the results of the RPMA analysis, we validated a pick of marker proteins found dysregulated in the mouse brain samples by Western blot, demonstrating a strong consistency with our high-throughput approach (Fig. 3a, b). This includes the downregulation of the phosphorylated forms of AKT, ERK, GSK3 and JNK. To farther dissect the apparently downregulated AKT/mTOR pathway, we investigated boosted proteins namely total and phosphorylated levels of mTOR itself and its upstream inhibitor AMPKβ1. Western blot analysis showed a trend towards a reduction of the AKT and p70S6K-mediated activating phosphorylation of mTOR at Ser2448, and significantly increased levels of pSer108-AMPKb1 (Fig. 3c, d), both confirming rather inhibitory furnishings on the mTOR pathway by the expression of expanded ataxin-3 [21,23].
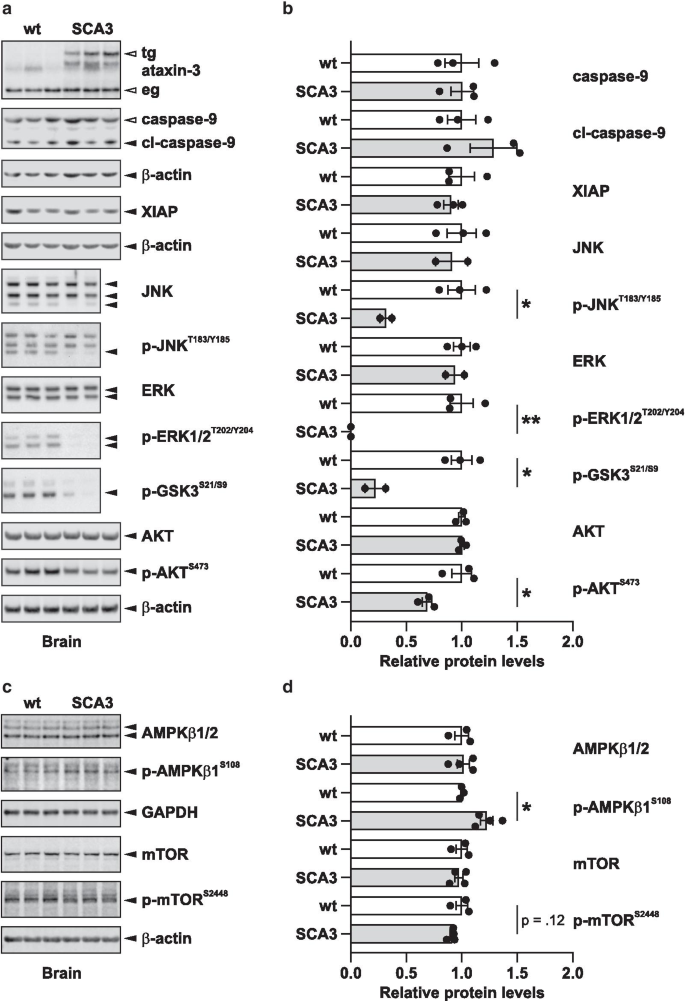
Confirmation of RPMA data by Western absorb analysis. For validation of RPMA information, Western blot analyses of mouse brains of SCA3 and wild-type mice were performed for selected targets. The same antibodies every bit in the RPMA analysis were used. Blot images (a) and graphs (b) showing their quantification. Quantified bands are marked with a black arrow. β-actin was used as loading control. In the SCA3 mouse model, transgenic expanded ataxin-iii (tg) is detected in addition to the endogenous ataxin-3 (eg). In improver, total and phosphorylated levels of AMPKβ1 and mTOR were analyzed (c and d) to further dissect the impact of expanded ataxin-3 on the mTOR signaling pathway. The confined correspond to means (wt n = 3, SCA3 due north = 2–four) ± SEM. Statistical meaning differences (t-examination) are marked (*, p < 0.05; **, p < 0.01)
Discussion
Polyglutamine expanded ataxin-iii decreases pro-survival signaling via the AKT pathway
The serine/threonine-protein kinase AKT is a key protein kinase involved in survival and growth with physiological links to neurological disorders. The levels of Serine 473-phosphorylated AKT (p-AKTS473), representing an active form of this poly peptide, were decreased in all iii models indicating a drop in pro-survival signaling due to the 148Q expansion. AKT signaling cascades include a number of proteins previously implicated in SCA3 such every bit GSK3, FOXO and mTOR [4, 18, 27, 42]. An increased susceptibility towards oxidative stress (via FOXO4 and SOD2) was suggested to exist a potential contributor to neuronal jail cell death in SCA3 and AKT, ERK, and JNK are all amongst the known kinases which target FOXOs and contribute to their activation and localization [4, 27].
Upstream of AKT lies the PI3K pathway. This pathway is counterbalanced and activated by the tumor suppressor phosphatase and tensin homolog PTEN. In our analyses, we observed an increment of both the total and phosphorylated levels of PTEN in SCA3 mouse encephalon samples and a subtract in HEK293T and MEF cells expressing expanded ataxin-3. Interestingly, ataxin-iii is suggested to exist responsible for repressing PTEN transcription in cancer [42] which further emphasizes the importance of the AKT signaling pathway in disease progression.
Altered GSK3 signaling in SCA3 models
Importantly, we besides saw a reduction of phosphorylated glycogen synthase kinase 3 (GSK3) shared among all SCA3 models investigated. GSK3α and GSK3β are constitutively agile, proline-directed serine/threonine kinases involved in a variety of cellular processes including glycogen metabolism [58], gene transcription [fifty], apoptosis [51] and microtubule stability [3, 13]. GSK3 is known to play a role in several neurological disorders [30]. With respect to SCA3 information technology is known to phosphorylate ataxin-3 and thus regulate its aggregation propensity [19, 37]. Furthermore, the phosphorylation of ataxin-3 by GSK3 contributes to its nuclear translocation [37], a process increasing the toxicity of expanded ataxin-3 and required for the manifestation of the disease [9, 48]. The reduction of GSK3 may therefore reflect a protective mechanism against this increase of toxicity.
GSK3 was besides the first reported substrate of AKT and the two are functionally similar. GSK3 is a potent promoter of Toll-like receptors-induced product of proinflammatory cytokines such as IL-6 which was found to exist altered in SCA3 patient samples [eighteen]. AKT is known to exert an inhibitory function on GSK3 via phosphorylation of Ser21/Ser9 (p-GSK3S21/S9) [22]. Consistent with the observed subtract in p-AKTS473, nosotros detected a reduced inhibitory phosphorylation of GSK3 in HEK293T and MEF cells expressing expanded ataxin-3.
Furthermore, GSK3 is required for activation of STAT proteins in astrocytes, microglia and macrophages [8]. Accordingly, our SCA3 cell models showed changes in seven proteins compared to two STAT proteins in the brain samples, while MEFKO cells showed profound alterations of STAT levels in response to the absence of ataxin-3 (Additional file 1: Table S1 and Fig. 2). These observations reinforce the link between ataxin-3 and GSK3 and should prompt the field to consider the role of insulin and glucose in encephalon homeostasis and neuronal degeneration as a metabolic syndrome. It is noteworthy, that GSK3 inhibitors recently gained attending in terms of their potential for diabetes, cancer, and neurodegeneration [33] highlighting a therapeutic potential for SCA3.
SCA3 models showroom alterations of AKT-associated factors
Tumor suppressor protein p53 is considered the "guardian of the genome" [28] because of its important role in determining cell fate. A tight regulation of p53 is critical in maintaining homeostasis and keeping a balance between its cancer-suppressive and age-promoting functions [10]. The interaction between p53 and GSK3 is hypothesized to be involved in aggregate clearance and neurodegeneration: the Alzheimer's disease hypothesis suggests that under normal conditions, GSK3 is involved in keeping p53 levels low while under conditions of cellular stress (e.k. aggregation) p53 is stabilized, proteasomal function declines, and levels of p53 increase [40, 51]. We observed that the levels of activated p-p53S15 increased in MEF148Q cells and in SCA3 mouse brains in contrast to its suppression by wild-type ataxin-3 (increase in MEFKO cells). Recently, p53 was identified equally a substrate of wild-type ataxin-three and the polyglutamine expansion was associated to increased p53-dependent neuronal expiry [31]. One tin hypothesize that SCA3 and other neurodegenerative disorders involve a positive feedback between proteasomal degradation, assemblage, GSK3 activation, and increased p53 levels. Likewise p53, the strongest increment in MEF cells both as reaction to the expression of expanded ataxin-3 and to the loss of ataxin-3 were observed for HSP90 and SUMO 2/iii. Both proteins were implicated before in SCA3 [2, 47] and our results further stress their relevance. Ataxin-3 is SUMOylated and the SUMOylation of ataxin-three regulates its function [ii]. Moreover, the inhibition of HSP90 turned out as promising therapeutic strategy in a mouse model for SCA3 [47].
The extracellular point regulated kinase ERK is a member of the MAPK pathway. It is a central protein lying at the intersection of proliferation, differentiation, and survival [29]. It was previously shown that ERK is responsible for the calcium-dependent phosphorylation of ataxin-i [26] and the downregulation of ERK reduces levels of ataxin-1 and suppresses neurodegeneration in Drosophila and mice [20]. ERK is further able to human action on downstream pathways of AKT and mTOR via control of the p70S6 kinase and the S6 ribosomal subunit [34]. The phosphorylated ERK (p-ERK1/twoT202/Y204) was reduced in all models tested including the knockout MEFKO cells. The latter suggested that the stimulating outcome of wild-type ataxin-3 in regulating p-ERK1/2T202/Y204 levels was abrogated by the polyglutamine expansion. We also institute that like to ERK, another MAPK, stress-activated p-JNKT183/Y185 displayed a strong inhibition by the expanded ataxin-three.
An boosted target of AKT is the endothelial nitric oxide synthase (eNOS) which catalyzes the production of nitric oxide. We observed a decrease in eNOS activation by ataxin-3 148Q in all models like to ERK. The phosphorylation of eNOS at Ser1177 (p-eNOSS1177) past AKT activates this enzyme. In terms of neurodegeneration, alterations in eNOS accept been linked to blood–encephalon barrier integrity [vi] which directly points to the importance of peripheral inflammation in propagation of CNS dysfunction.
Indication of dumb mTOR activation in SCA3 models
P70S6 kinase is a mitogen-activated kinase affected by ERK signaling downstream of mTOR and AKT. It phosphorylates the S6 poly peptide of the 40S ribosomal subunit (RPS6) and thus controls mRNA translation. The phosphorylation of RPS6 is commonly used as a readout of mTOR activation [36] and other piece of work suggests that the state of Ser240/Ser244 phosphorylation of RPS6 (p-RPS6S240/S244) tin be used to estimate the neuronal action land of striatal cholinergic interneurons [7]. We observed decreased levels of p-RPS6S240/S244 in all models and MEFKO cells indicating a negative affect of the presence of expanded ataxin-3 on mTOR activation. Moreover, an interaction betwixt ataxin-iii and the kinase (ribosomal poly peptide S6 kinase alpha-one) phosphorylating RPS6 has also been suggested [1, 52].
Forth with p70S6K, another important neuron-related kinase continued with mTOR is Src. Our data indicate an increased activity of Src in our models: The Tyr527 phosphorylation site of Src (p-SrcY527) which renders the enzyme less active was decreased in HEK148Q, MEF148Q and mouse brain samples. Although first identified equally a proto-oncogene, Src was found to be expressed in differentiated, post-mitotic neurons and is important for neuronal differentiation and neurite outgrowth with the capability to command ion aqueduct activity and synaptic manual [43]. Src is involved in the intracellular release of calcium stores from the endoplasmic reticulum [53]. Concordant with our information, a reduced phophorylation of Src has likewise been observed in a unlike mouse model of SCA3 [59]. Moreover, Src has been linked to Huntington's disease where expression of polyglutamine-expanded huntingtin activates Src and ultimately promotes neuronal expiry induced by glutamate. In this line, Src was constitute to be altered in ataxin-two patient fibroblasts and ataxin-two knockout lines [16].
Further supporting our RPMA analysis, we observed using Western blots a trend towards reduced phosphorylation of mTOR at Ser2448, an activating modification mediated by AKT and p70S6K [23], and significantly increased levels of Ser108-phosphorylated AMPKβ1, which acts as an upstream inhibitor of mTOR [21, 54]. These observations back up the indication of rather impaired mTOR activation. In an earlier study, the inhibition of mTOR led to an induction of autophagy thereby ameliorating the toxicity of expanded ataxin-3 in SCA3 mice [35]. Consistently, increasing phosphorylated AMPK by the administration of cordycepin in SCA3 cells and mice was shown to have neuroprotective furnishings via activation of autophagy and lowering mutant poly peptide synthesis [32]. Thus, these findings suggest that the detected lowering of mTOR activation in our models may correspond a rescue machinery.
Pro-survival, anti-apoptotic contribution of polyglutamine expansion
Apoptosis and necrosis are believed to be the two major death pathways for neurons [fourteen]. Regarding apoptosis, it was suggested that initiator or executor caspases are activated in neurodegenerative diseases [57]. In Huntington'southward disease, neurons positive for caspase-3 dice quickly but for those with aggregates trigger cellular quiescence, deactivate apoptosis but actuate delayed necrosis, which supports the argument that high-ordered aggregates or inclusions might be protective [41]. In our assay, the levels of broken caspases including caspase-iii, -7, and -ix were increased in SCA3 mouse brains while either decreased or not altered in MEF and HEK293T cell models. Seemingly contradictory behavior was displayed by the pro-apoptotic FADD (activated in MEF148Q cells, but suppressed in our other models). Bcl-two family members (Bcl-twoscore, Bax, Bad, Bim) besides as FLIP, SHIP and XIAP were uniformly either reduced or not altered. Recent information showed that the ratio of Bcl2/Bax was decreased in SCA3 patients compared to controls, suggesting that the ratios and balances of these proteins are perhaps more than important than their accented values [61]. Consistent with this, comparison of normal vs. expanded ataxin-3 showed that the presence of the polyglutamine expansion within ataxin-3 provided a pro-survival and anti-apoptotic contribution. Since the cell behavior is dictated by a superposition of multiple signals, the overall rest in favor of survival or death outcomes cannot be predicted with certainty based on our information simply. Nevertheless, it seems reasonable to conclude that, depending on the particular cellular context, the expanded ataxin-3 can provide a widespread anti-apoptotic impact on cell signaling.
Determination
In our work, we used RPMA analysis to narrate the altered signaling networks observed upon ataxin-three expansion in both in vitro and in vivo models. It is important to consider that these models take big differences such as innate expression of ataxin-3, overexpression of ataxin-3, have unlike levels of sensitivity to expanded protein and are derived from different tissues. Nosotros employed a set of antibodies which comprehend various pathways across the cellular landscape including inflammation, survival, free energy metabolism, growth, transcription, translation, apoptosis, mitochondrial integrity and autophagy. We suggest that elucidating these signaling cascades is integral in understanding the function of ataxin-iii in health and disease. Nosotros demonstrate that across our models, AKT/mTOR pathway targets (Table i) are significantly contradistinct. Our results revealed alterations shared betwixt tested models as well as the unique differences providing new perspectives on altered signaling in SCA3. In hereafter studies, the role of the altered pathways needs to be correlated to the disease progression and outcome in SCA3 patients and beast models [46, 49]. This analysis can shed calorie-free on future targets for inquiry and pharmaceutical development by painting a clearer film of the neurodegenerative landscape.
Availability of information and materials
All data generated or analyzed during this written report are included in this published article and its supplementary information.
Change history
-
22 May 2021
The Open Admission funding statement has been updated in this publication.
Abbreviations
- SCA3:
-
Spinocerebellar ataxia type 3
- MJD:
-
Machado-Joseph affliction
- AKT:
-
AKT8 virus oncogene cellular homolog
- ATF:
-
Activating transcription gene 2
- Bad:
-
Bcl-2-associated agonist of prison cell death
- Bax:
-
Bcl-two-associated X protein
- Bcl-xL:
-
B-prison cell lymphoma-extra big
- Bim:
-
Bcl-ii-interacting mediator
- cl:
-
Prefix cl- denotes a cleaved grade
- CREB:
-
Military camp response chemical element-binding poly peptide
- eNOS:
-
Endothelial nitric oxide synthase
- ERK:
-
Extracellular betoken-regulated kinase
- FADD:
-
Fas-associated protein with death domain
- FLIP:
-
FLICE (Caspase-8) inhibitory poly peptide
- FOXO:
-
Forkhead box protein class O4
- GAPDH:
-
Glyceraldehyde-three-phosphate dehydrogenase
- GSK:
-
Glycogen synthase kinase
- HSP27:
-
Rut shock protein 27
- HSP90:
-
Heat stupor protein ninety
- IκBα:
-
Inhibitor of NF-κB blastoff subunit
- IL-10:
-
Interleukin 10
- JNK:
-
Jun Northward-final kinase
- mTOR:
-
Mammalian target of rapamycin
- NF-κB:
-
Nuclear factor κB
- p-:
-
Prefix p- denotes a phosphorylated protein
- p53:
-
Tumor suppressor protein that protects from DNA harm
- p70S6K:
-
Phosphoprotein seventy ribosomal protein S6 kinase
- PARP:
-
Poly(ADP-ribose) polymerase
- PTEN:
-
Phosphatase and tensin homolog deleted on chromosome x
- RPMA:
-
Reverse-phase Protein MicroArray
- RPS6:
-
Small-subunit ribosomal poly peptide S6
- Transport:
-
Src homology 2 (SH2) domain-containing inositol phosphatase
- Src:
-
Rous sarcoma oncogene cellular homolog
- STAT:
-
Signal transducer and activator of transcription
- SUMO:
-
Small ubiquitin-like modifier
- TNF-R1:
-
Tumor necrosis factor receptor 1
- wt:
-
Wild-type
- XIAP:
-
Ten-linked inhibitor of apoptosis
References
-
Almeida B, Fernandes S, Abreu IA, Macedo-Ribeiro Due south. Trinucleotide repeats: a structural perspective. Front Neurol. 2013;4:76. https://doi.org/10.3389/fneur.2013.00076.
-
Almeida B, Abreu IA, Matos CA, Fraga JS, Fernandes S, Macedo MG, Gutiérrez-Gallego R, Pereira PJ, Carvalho AL, Macedo-Ribeiro S. SUMOylation of the brain-predominant Ataxin-3 isoform modulates its interaction with p97. Biochem Biophys Acta. 2015;1852(9):1950–ix. https://doi.org/10.1016/j.bbadis.2015.06.010.
-
Anderton BH, Betts J, Blackstock WP, Brion JP, Chapman South, Connell J, et al. Sites of phosphorylation in tau and factors affecting their regulation. Biochem Soc Symp. 2001;67:73–80.
-
Araujo J, Breuer P, Dieringer S, Krauss S, Dorn Southward, Zimmermann K, et al. FOXO4-dependent upregulation of superoxide dismutase-2 in response to oxidative stress is impaired in spinocerebellar ataxia type three. Hum Mol Genet. 2011;twenty(xv):2928–41. https://doi.org/x.1093/hmg/ddr197.
-
Ardito F, Giuliani M, Perrone D, Troiano Thousand, Lo Muzio Fifty. The crucial role of protein phosphorylation in jail cell signaling and its apply as targeted therapy (Review). Int J Mol Med. 2017;forty(2):271–80. https://doi.org/10.3892/ijmm.2017.3036.
-
Beauchesne É, Desjardins P, Hazell Equally, Butterworth RF. ENOS gene deletion restores blood-encephalon barrier integrity and attenuates neurodegeneration in the thiamine-deficient mouse brain. J Neurochem. 2009;111(two):452–nine. https://doi.org/10.1111/j.1471-4159.2009.06338.x.
-
Bertran-Gonzalez J, Chieng BC, Laurent V, Valjent Due east, Balleine BW. Striatal cholinergic interneurons display activity-related phosphorylation of ribosomal protein S6. PLoS ONE. 2012;seven:12. https://doi.org/10.1371/journal.pone.0053195.
-
Beurel Due east, Jope RS. Differential regulation of STAT family members past glycogen synthase kinase-3. J Biol Chem. 2008;283(32):21934–44. https://doi.org/x.1074/jbc.M802481200.
-
Bichelmeier U, Schmidt T, Hübener J, Boy J, Rüttiger L, Häbig Thousand, Poths S, Bonin G, Knipper Thou, Schmidt WJ, Wilbertz J, Wolburg H, Laccone F, Riess O. Nuclear localization of ataxin-3 is required for the manifestation of symptoms in SCA3: in vivo bear witness. J Neurosci. 2007;27(28):7418–28. https://doi.org/x.1523/JNEUROSCI.4540-06.2007.
-
Bieging KT, Mello SS, Attardi LD. Unravelling mechanisms of p53-mediated neoplasm suppression. Nat Rev Cancer. 2014;14(5):359–70. https://doi.org/10.1038/nrc3711.
-
Boy J, Schmidt T, Wolburg H, Mack A, Nuber South, Böttcher Grand, et al. Reversibility of symptoms in a conditional mouse model of spinocerebellar ataxia type 3. Human Mol Genetics. 2009;18(22):4282–95. https://doi.org/10.1093/hmg/ddp381.
-
Boy J, Schmidt T, Schumann U, Grasshoff U, Unser S, Holzmann C, et al. A transgenic mouse model of spinocerebellar ataxia blazon three resembling late disease onset and gender-specific instability of CAG repeats. Neurobiol Dis. 2010;37(2):284–93. https://doi.org/ten.1016/j.nbd.2009.08.002.
-
Brion JP, Anderton BH, Authelet G, Dayanandan R, Leroy K, Lovestone Southward, et al. Neurofibrillary tangles and tau phosphorylation. Biochem Soc Symp. 2001;67:81–eight.
-
Chi H, Chang H-Y, Sang T-K. Neuronal Jail cell Decease Mechanisms in Major Neurodegenerative Diseases. Int J Mol Sci. 2018;19(10):3082. https://doi.org/10.3390/ijms19103082.
-
Chou A-H, Lin A-C, Hong M-Y, Hu Southward-H, Chen Y-L, Chen J-Y, et al. p53 activation mediates polyglutamine-expanded ataxin-iii upregulation of Bax expression in cerebellar and pontine nuclei neurons. Neurochem Int. 2011;58(ii):145–52. https://doi.org/10.1016/j.neuint.2010.11.005.
-
Drost J, Nonis D, Eich F, Leske O, Damrath E, Brunt ER, et al. Ataxin-two modulates the levels of Grb2 and SRC but not ras signaling. J Mol Neurosci. 2013;51(1):68–81. https://doi.org/10.1007/s12031-012-9949-four.
-
Einspahr JG, Calvert V, Alberts DS, Curiel-Lewandrowski C, Warneke J, Krouse R, et al. Functional poly peptide pathway activation mapping of the progression of normal skin to squamous cell carcinoma. Cancer Prev Res. 2012;5(3):403–13. https://doi.org/10.1158/1940-6207.CAPR-11-0427.
-
Evert BO, Schelhaas J, Fleischer H, de Vos R, a I., Brunt, East. R., Stenzel, W., , et al. Neuronal intranuclear inclusions, dysregulation of cytokine expression and cell decease in spinocerebellar ataxia type 3. Clin Neuropathol. 2006;25(six):272–81.
-
Fei Due east, Jia N, Zhang T, Ma 10, Wang H, Liu C, et al. Phosphorylation of ataxin-3 past glycogen synthase kinase 3β at serine 256 regulates the assemblage of ataxin-3. Biochem Biophys Res Commun. 2007;357(ii):487–92. https://doi.org/ten.1016/j.bbrc.2007.03.160.
-
Ferrarelli LK. Conserved signals in neurodegeneration. Sci Signal. 2013;half-dozen(281):143. https://doi.org/x.1126/scisignal.2004445.
-
Garcia D, Shaw RJ. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol Prison cell. 2017;66(6):789–800. https://doi.org/10.1016/j.molcel.2017.05.032.
-
Hermida MA, Dinesh Kumar J, Leslie NR. GSK3 and its interactions with the PI3K/AKT/mTOR signalling network. Adv Biol Regul. 2017;65:v–15. https://doi.org/x.1016/j.jbior.2017.06.003.
-
Hoeffer CA, Klann Due east. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33(ii):67–75. https://doi.org/10.1016/j.tins.2009.11.003.
-
Hübener J, Vauti F, Funke C, Wolburg H, Ye Y, Schmidt T, et al. N-terminal ataxin-three causes neurological symptoms with inclusions, endoplasmic reticulum stress and ribosomal dislocation. Brain. 2011;134(7):1925–42. https://doi.org/10.1093/encephalon/awr118.
-
Kawaguchi Y, Okamoto T, Taniwaki M, Aizawa M, Inoue M, Katayama S, et al. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q321. Nature Genet. 1994;8(3):221–8.
-
Kaytor Medico, Byam CE, Tousey SK, Stevens SD, Zoghbi HY, et al. A cell-based screen for modulators of ataxin-1 phosphorylation. Hum Mol Genet. 2005;14(8):1095–105. https://doi.org/ten.1093/hmg/ddi122.
-
Klotz LO, Sánchez-Ramos C, Prieto-Approach I, Urbánek P, Steinbrenner H, Monsalve M. Redox regulation of FoxO transcription factors. Redox Biol. 2015. https://doi.org/10.1016/j.redox.2015.06.019.
-
Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358(6381):15–6. https://doi.org/x.1038/358015a0.
-
Lavoie H, Gagnon J, Therrien Chiliad. ERK signalling: a principal regulator of cell behaviour, life and fate. Nat Rev Mol Cell Biol. 2020;21(10):607–32. https://doi.org/10.1038/s41580-020-0255-vii.
-
Lei P, Ayton Southward, Bush-league AI, Adlard PA. GSK-3 in neurodegenerative diseases. Int J Alzheimer's Dis. 2011;2011:1–ix. https://doi.org/ten.4061/2011/189246.
-
Liu H, Li X, Ning Yard, Zhu S, Ma X, Liu X, et al. The Machado-Joseph Disease Deubiquitinase Ataxin-3 regulates the stability and apoptotic function of p53. PLoS Biol. 2016;14(eleven):e2000733. https://doi.org/10.1371/journal.pbio.2000733.
-
Marcelo A, Brito F, Carmo-Silva S, Matos CA, Alves-Cruzeiro J, Vasconcelos-Ferreira A, Koppenol R, Mendonça L, de Almeida LP, Nóbrega C. Cordycepin activates autophagy through AMPK phosphorylation to reduce abnormalities in Machado-Joseph disease models. Hum Mol Genet. 2019;28(1):51–63. https://doi.org/10.1093/hmg/ddy328.
-
Maqbool Thousand, Hoda North. GSK3 inhibitors in the therapeutic development of diabetes, cancer and Neurodegeneration: by, present and future. Curr Pharm Des. 2017;23(29):4332–50. https://doi.org/10.2174/1381612823666170714141450.
-
Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci. 2011;36(six):320–8. https://doi.org/x.1016/j.tibs.2011.03.006.
-
Menzies FM, Huebener J, Renna M, Bonin Thou, Riess O, Rubinsztein DC. Autophagy induction reduces mutant ataxin-3 levels and toxicity in a mouse model of spinocerebellar ataxia type three. Encephalon. 2010;133(Pt ane):93–104. https://doi.org/10.1093/brain/awp292.
-
Meyuhas O. Ribosomal protein S6 phosphorylation. Int Rev Cell Mol Biol. 2015;320:41–73. https://doi.org/10.1016/bs.ircmb.2015.07.006.
-
Mueller T, Breuer P, Schmitt I, Walter J, Evert BO, Wüllner U. CK2-dependent phosphorylation determines cellular localization and stability of ataxin-iii. Hum Mol Genet. 2009;eighteen:3334–43. https://doi.org/x.1093/hmg/ddp274.
-
Popova TG, Espina V, Zhou W, Mueller C, Liotta Fifty, Popov SG. Whole proteome analysis of mouse lymph nodes in cutaneous anthrax. PLoS 1. 2014;9(10):e110873. https://doi.org/x.1371/journal.pone.0110873.
-
Popova TG, Espina V, Liotta LA, Popov SG. Reverse-phase microarray analysis reveals novel targets in lymph nodes of bacillus anthracis spore-challenged mice. PLoS ONE. 2015;x(6):e0129860. https://doi.org/10.1371/journal.pone.0129860.
-
Proctor CJ, Gray DA. GSK3 and p53 - is there a link in Alzheimer'south disease? Mol Neurodegen. 2010;5(vii):1–15. https://doi.org/ten.1186/1750-1326-5-seven.
-
Ramdzan YM, Trubetskov MM, Ormsby AR, Newcombe EA, Sui X, Tobin MJ, et al. huntingtin inclusions trigger cellular quiescence, deactivate apoptosis, and lead to delayed necrosis. Cell Reports. 2017;19(v):919–27. https://doi.org/10.1016/j.celrep.2017.04.029.
-
Sacco JJ, Yau TY, Darling S, Patel 5, Liu H, Urbé South, et al. The deubiquitylase Ataxin-three restricts PTEN transcription in lung cancer cells. Oncogene. 2013. https://doi.org/10.1038/onc.2013.512.
-
Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5(4):317–28. https://doi.org/ten.1038/nrn1368.
-
Schmidt J, Mayer AK, Bakula D, Freude J, Weber JJ, Weiss A, et al. Vulnerability of frontal brain neurons for the toxicity of expanded ataxin-3. Hum Mol Genet. 2019;28(nine):1463–73. https://doi.org/ten.1093/hmg/ddy437.
-
Schmitt I, Linden M, Khazneh H, Evert BO, Breuer P, Klockgether T, et al. Inactivation of the mouse Atxn3 (ataxin-iii) gene increases protein ubiquitination. Biochem Biophys Res Commun. 2007;362:734–9. https://doi.org/ten.1016/j.bbrc.2007.08.062.
-
Shi C-SS, Shenderov K, Huang N-NN, Kabat J, Abu-Asab Yard, Fitzgerald KA, et al. Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol. 2012;xiii(three):255–63. https://doi.org/10.1038/ni.2215.
-
Silva-Fernandes A, Duarte-Silva Southward, Neves-Carvalho A, Amorim M, Soares-Cunha C, Oliveira P, Thirstrup M, Teixeira-Castro A, Maciel P. Chronic treatment with 17-DMAG improves balance and coordination in a new mouse model of Machado-Joseph illness. Neurotherapeutics. 2014;eleven(2):433–49. https://doi.org/x.1007/s13311-013-0255-9.
-
Sowa Equally, Martin E, Martins IM, Schmidt J, Depping R, Weber JJ, et al. Karyopherin α-3 is a central protein in the pathogenesis of spinocerebellar ataxia type 3 decision-making the nuclear localization of ataxin-three. Proc Natl Acad Sci. 2018;115(xi):E2624–33. https://doi.org/10.1073/pnas.1716071115.
-
Spooren A, Kolmus K, Laureys Thousand, Clinckers R, De Keyser J, Haegeman G, et al. Interleukin-half-dozen, a mental cytokine. Brain Res Rev. 2011. https://doi.org/ten.1016/j.brainresrev.2011.01.002.
-
Troussard AA, Tan C, Yoganathan TN, Dedhar S. Jail cell-extracellular matrix interactions stimulate the AP-1 transcription factor in an integrin-linked kinase- and glycogen synthase kinase 3-dependent manner. Mol Cell Biol. 1999;xix:7420–7.
-
Turenne GA, Toll BD. Glycogen synthase kinase3 beta phosphorylates serine 33 of p53 and activates p53'south transcriptional activity. BMC Prison cell Biol. 2001;two:12. https://doi.org/x.1186/1471-2121-2-12.
-
Vinayagam A, Stelzl U, Foulle R, Plassmann S, Zenkner M, Timm J, et al. A directed protein interaction network for investigating intracellular bespeak transduction. Sci Signal. 2011;iv(189):rs8. https://doi.org/10.1126/scisignal.2001699.
-
Wang H, Reiser One thousand. The part of the Ca two+-sensitive tyrosine kinase Pyk2 and Src in thrombin signalling in rat astrocytes. J Neurochem. 2003;84(half-dozen):1349–57. https://doi.org/10.1046/j.1471-4159.2003.01637.10.
-
Warden SM, Richardson C, O'Donnell J Jr, Stapleton D, Kemp BE, Witters LA. Post-translational modifications of the beta-i subunit of AMP-activated protein kinase affect enzyme activity and cellular localization. Biochem J. 2001;354(Pt 2):275–83. https://doi.org/ten.1042/0264-6021:3540275.
-
Weber JJ, Sowa AS, Binder T, Hübener J. From pathways to targets: agreement the mechanisms behind polyglutamine affliction. Biomed Res Int. 2014. https://doi.org/ten.1155/2014/701758.
-
Weber JJ, Haas E, Maringer Y, Hauser Due south, Casadei NLP, Chishti AH, Riess O, Hübener-Schmid J. Calpain-ane ablation partially rescues illness-associated hallmarks in models of Machado-Joseph illness. Hum Mol Genet. 2020;29(6):892–906. https://doi.org/x.1093/hmg/ddaa010.
-
Wellington CL, Hayden MR. Caspases and neurodegeneration: on the cutting edge of new therapeutic approaches. Clin Genet. 2000;57(1):1–ten. https://doi.org/10.1034/j.1399-0004.2000.570101.ten.
-
Welsh GI, Proud CG. Glycogen synthase kinase-3 is rapidly inactivated in response to insulin and phosphorylates eukaryotic initiation factor eIF-2B. Biochem J. 1993;294(Pt 3):625–9.
-
Wiatr K, Piasecki P, Marczak Ł, Wojciechowski P, Kurkowiak M, Płoski R, Rydzanicz M, Handschuh L, Jungverdorben J, Brüstle O, Figlerowicz M, Figiel K. Altered levels of proteins and phosphoproteins, in the absence of early causative transcriptional changes, shape the molecular pathogenesis in the brain of immature presymptomatic Ki91 SCA3/MJD Mouse. Mol Neurobiol. 2019;56(12):8168–202. https://doi.org/10.1007/s12035-019-01643-4.
-
Wilson B, Liotta LA, Petricoin Due east tertiary. Monitoring proteins and protein networks using reverse phase poly peptide arrays. Dis Markers. 2010;28(4):225–32. https://doi.org/10.3233/DMA-2010-0705.
-
Yang Z, Shi C, Zhou 50, Li Y, Yang J, Liu Y, et al. Metabolic profiling reveals biochemical pathways and potential biomarkers of spinocerebellar clutter 3. Forepart Mol Neurosci. 2019;12:159. https://doi.org/10.3389/fnmol.2019.00159.
Acknowledgements
We want to thank Virginia Espina for her technical assistance on this project and Ina Schmitt for providing ataxin-3 knockout mice. We further acknowledge support by the Open Access Publishing Fund of the Eberhard Karls University of Tuebingen.
Funding
Open Access funding enabled and organized by Projekt DEAL. The work leading to this invention has received funding from the European Commission'south 7th Framework Programme FP7/2010 under grant agreement no. 264508 (TreatPolyQ) and the Federal Ministry of Education and Inquiry (PPPT-MJD, grant agreement no. 01GM1309B; SCA-CYP, grant understanding no. 01GM1803) under the umbrella of E-Rare (ERA-Net for Research Programmes on Rare Diseases).
Author information
Affiliations
Contributions
Conceptualization, ASS and TP; Investigation, Donkey, TSP, Thursday, JJW, JS, PSS; Writing-Original Typhoon, Ass; Writing-Review & Editing, ASS, JHS, JJW, JS, TS; Supervision, JHS, TS; Funding Acquisition, TS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The written report was carried out in strict accordance with the recommendations presented in the Guide of Intendance and Use of Laboratory Animals of the University of Tuebingen, Germany. The protocols were approved past the Institutional Brute Intendance and Use Committee (IACUC) of the University of Tuebingen, Germany.
Consent for publication
Not applicable.
Competing interests
The authors declare that they accept no competing interests.
Additional information
Publisher's Notation
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, equally long every bit you give advisable credit to the original author(southward) and the source, provide a link to the Artistic Commons licence, and indicate if changes were fabricated. The images or other third political party cloth in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is non included in the article'southward Artistic Commons licence and your intended apply is not permitted by statutory regulation or exceeds the permitted employ, you will need to obtain permission direct from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Artistic Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the information made available in this commodity, unless otherwise stated in a credit line to the information.
Reprints and Permissions
Almost this article
Cite this commodity
Sowa, A.S., Popova, T.G., Harmuth, T. et al. Neurodegenerative phosphoprotein signaling mural in models of SCA3. Mol Brain xiv, 57 (2021). https://doi.org/10.1186/s13041-020-00723-0
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/ten.1186/s13041-020-00723-0
Keywords
- Spinocerebellar ataxia type 3 (SCA3)
- Machado-Joseph disease (MJD)
- Ataxin-iii (ATXN3)
- RPMA
- Neurodegeneration
- pERK
- AKT (PKB)
- mTOR
- Phosphoprotein
Which Statement About Creb327 Phosphorylation Is Supported By The Data Presented In Table 1?,
Source: https://molecularbrain.biomedcentral.com/articles/10.1186/s13041-020-00723-0
Posted by: martincouseed1937.blogspot.com


0 Response to "Which Statement About Creb327 Phosphorylation Is Supported By The Data Presented In Table 1?"
Post a Comment